The First. The Only.
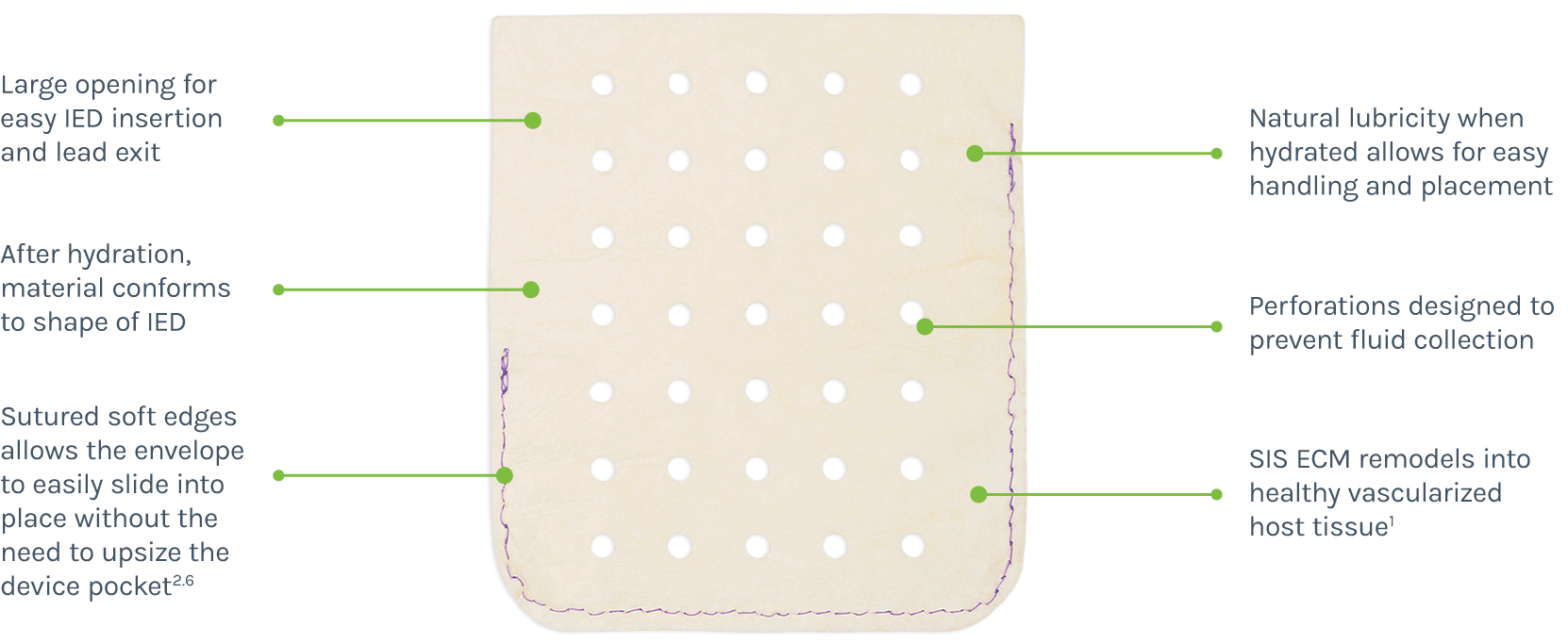
CanGaroo Envelope is comprised of acellular biologic material. Porcine small intestine submucosa extracellular matrix (SIS ECM): Designed to create a healthy pocket.1
How CanGaroo Works.
CanGaroo is comprised of extracellular matrix which promotes a natural biologic healing response resulting in healthy, vascularized tissue.3
Extracellular matrix regulates the biologic healing response to decrease inflammation and stimulate formation of healthy tissue.3,4 CanGaroo is an extracellular matrix that creates a hospitable environment for host cells to migrate and initiate tissue remodeling.1,5

- Regulates cell adhesion, differentiation, division, and migration.5
- Patients own cells migrate and integrate into the material.5

- Promotes natural wound healing mechanisms by developing structural tissue which matures to form a strong permanent repair.5
Image: Data on file at Aziyo Biologics, Inc.
CanGaroo Benefits.
Remodels into a healthy, vascularized pocket.3
Implantation
CanGaroo easily conforms to the shape of the CIED. Once implanted, CanGaroo Envelope creates a hospitable environment for the surrounding cells to migrate into the bioscaffold and start matrix turnover.1,5
Remodeling
The extracellular matrix promotes the biologic healing response triggered from the procedure to reduce inflammation and modulate healing.3,4
Within the New Tissue
As remodeling concludes, the CIED is secured naturally with the new tissue.3 The result is systemically connected vascularized tissue that surrounds the device.3
Representative product shown.
Case Image
Three Year Post CanGaroo Envelope Implant
82-year-old patient requiring re-operation for device upgrade.

Device Pocket on Re-entry

Device Pocket on Re-entry with CIED and Leads Removed
Photo courtesy of David Woodard, MD, FACC
Patients Who May Benefit from CanGaroo Envelope.
CanGaroo remodels into healthy, systemically vascularized tissue.3 This may be beneficial for the younger population who may have multiple change-outs in their lifetimes. Patients with thin skin may be at risk for erosion and may benefit from an added layer of tissue that secures the device.3 CanGaroo provides a soft layer around the device which helps anchor the CIED, naturally mitigating risk of migration and/or erosion.3 The CanGaroo may facilitate device implant by providing additional anchoring points.6 CanGaroo provides additional anchoring points at time of device implant resulting in long-term, natural stabilization.3,6 This increased stabilization may also protect both sensing and shocking vector.6 CanGaroo may facilitate abdominal implants by providing additional anchoring points.6 CanGaroo remodels into healthy, systemically vascularized tissue, which may help ease future change-outs.3 This is especially beneficial for the younger population which may have multiple change-outs in their lifetimes. SIS ECM has been shown to naturally resist calcification.5Clinical Challenge
How CanGaroo Envelope Helps
Visit Our New Clinical Insights and Expert Opinions Resource.
To see why leaders in Electrophysiology recommend CanGaroo.
Product Specifications
Sizing and Ordering Information
- Product Numbers are for U.S. only.
- For International Sales Contact Customer Service.
Available in 5 Sizes, Multiple Packaging Options, Extended Shelf Life
- Available in 2 packaging configurations, 2.5 year shelf life (30 months)
| wdt_ID | Size | Dimensions | Recommended Device Size |
|---|---|---|---|
| 1 | Small | 5.4cm x 5.0cm | Pediatric Pacemaker |
| 2 | Medium | 6.9cm x 6.5cm | Pacemaker |
| 3 | Large | 6.9cm x 8.0cm | ICD |
| 4 | X-Large | 6.9cm x 9.5cm | CRT-D and ICD |
| 5 | Sub-Q | 10.8cm x 8.9cm | S-ICD |
Ordering Information
| wdt_ID | Product Number | Size |
|---|---|---|
| 1 | CMCV–009–SML | CanGaroo® Envelope, S, Single Pack |
| 2 | CMCV–009-MED | CanGaroo® Envelope, M, Single Pack |
| 3 | CMCV–009-LRG | CanGaroo® Envelope, L, Single Pack |
| 4 | CMCV–009-XLG | CanGaroo® Envelope, XL, Single Pack |
| 5 | CMCV–009-XXL | CanGaroo® Envelope, XXL, Single Pack |
| 6 | CMCV–010–SML | CanGaroo® Envelope, S, Five Pack |
| 7 | CMCV–010-MED | CanGaroo® Envelope, M, Five Pack |
| 8 | CMCV–010-LRG | CanGaroo® Envelope, L, Five Pack |
| 9 | CMCV–010-XLG | CanGaroo® Envelope, XL, Five Pack |
| 10 | CMCV–010-XXL | CanGaroo® Envelope, XXL, Five Pack |
Download Instructions for Use (IFU)
IFU for U.S. only.
IFU for Outside U.S. only.
Try CanGaroo Envelope Today!
To schedule a product demo or request an in-service for a product evaluation contact us today.
The CanGaroo Envelope is intended to securely hold a cardiac implantable electronic device or implantable neurostimulator.
1. Data on file at Elutia Inc.
2. Piterina A, et al. Int J Mol Sci. 2009 Nov 20;10(10):4375-417.
3. Pre-clinical data on file at Elutia Inc.
4. Dziki JL, et al. J Biomed Mater Res A. 2017;105(1):138–147.
5. Sundermann SH, et al. Thorac Cardiovasc Surg. 2014 Feb;62(1):76-9.
6. Xiang K, et al. HeartRhythm Case Rep. 2019;5(8):430-432.
WMK-1669-01C